Enteromix: Russia’s AI-Powered Personalized mRNA Cancer Vaccine Heralding a New Era in Precision Oncology
The Future of Trials: How Artificial Intelligence Is Accelerating Personalized Cancer Vaccines
Enteromix, Russia’s groundbreaking personalized mRNA cancer vaccine, has captured global attention by reporting 100 percent safety and remarkable tumor regression in its initial human trials. By harnessing the flexibility of messenger RNA (mRNA) technology and coupling it with cutting-edge artificial intelligence (AI) for neoantigen selection, Enteromix promises to redefine cancer treatment as a model of precision immunotherapy.
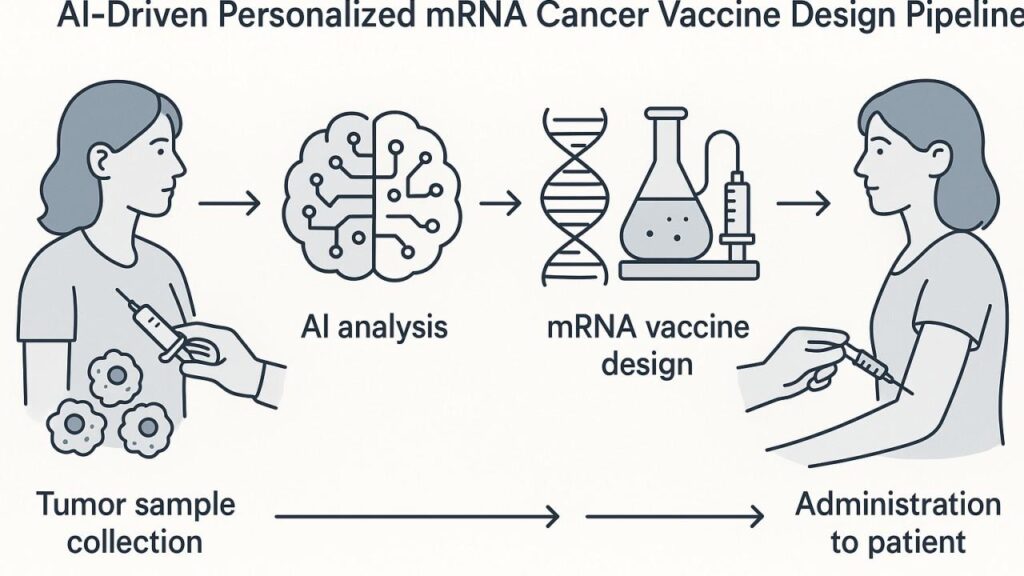
From Pandemic Defense to Cancer Eradication: The Rise of mRNA Vaccines
The success of mRNA vaccines against COVID-19 demonstrated that synthetic mRNA, encapsulated in lipid nanoparticles (LNPs), could instruct human cells to produce target proteins safely and effectively. This platform’s rapid adaptability and scalable manufacturing opened doors beyond infectious diseases into oncology. Unlike viruses, tumors harbor unique, patient-specific mutations called neoantigens, making them ideal targets for personalized vaccines. However, identifying which neoantigens will elicit a robust immune response requires massive data analysis—enter AI-driven bioinformatics.
The Precision Pipeline: How Enteromix Personalizes Cancer Immunotherapy
Enteromix’s development integrates five critical stages:
- Tumor Genomic Profiling A small biopsy from the patient’s tumor undergoes whole-exome sequencing, revealing hundreds of mutations. This genomic fingerprint lays the foundation for bespoke vaccine design.
- AI-Driven Neoantigen Prediction Sophisticated machine learning algorithms analyze mutation data alongside the patient’s human leukocyte antigen (HLA) type. By predicting peptide–HLA binding affinity and immunogenic potential, AI narrows thousands of candidates down to the top four to eight neoantigens most likely to activate CD8+ T cells.
- Synthetic mRNA Design and Optimization Once neoantigens are selected, generative AI models optimize codon usage and mRNA secondary structure to enhance stability and translational efficiency. Modified nucleosides—such as pseudouridine—are incorporated to reduce innate immune sensing and improve protein expression.
- Lipid Nanoparticle Formulation The engineered mRNA is encapsulated in an LNP blend—ionizable lipids, DSPC, cholesterol, and PEG-lipids—designed for optimal endosomal escape and targeted delivery to dendritic cells in lymph nodes. Each particle measures approximately 100 nanometers, ensuring efficient cellular uptake after intramuscular injection.
- Rapid Manufacturing and Quality Control Through AI-guided automation, Enteromix’s synthesis pipeline compresses the timeline from tumor sequencing to vaccine batch release to just seven to ten days. Real-time analytics monitor the capping reaction, mRNA integrity, and LNP assembly, maintaining batch failure rates below 2 percent.
Preclinical Foundations: Demonstrating Efficacy and Safety
Before entering clinical trials, Enteromix underwent extensive evaluation in murine models of colorectal and melanoma cancers. In these preclinical studies, tumor volumes shrank by 60–80 percent, and treated mice exhibited a 50 percent increase in median survival compared with controls. Immune profiling revealed robust infiltration of neoantigen-specific CD8+ T cells and elevated interferon-gamma release, validating both the AI selection process and the LNP delivery system.
Phase I Results: A Milestone in Personalized Oncology
In early 2025, Enteromix entered a Phase I clinical trial involving 48 patients with advanced colorectal cancer who had exhausted standard therapies. Three intramuscular doses were administered at two-week intervals. The safety profile was impeccable: no serious adverse events were observed, and mild injection-site pain, transient fever, and fatigue were self-limited.
Remarkably, complete tumor regressions occurred in a subset of patients, while over 40 percent experienced partial responses with tumor shrinkage exceeding 50 percent. An additional 30 percent achieved stable disease lasting more than six months. Correlative biomarker analyses confirmed that patients with the highest frequencies of neoantigen-specific T cells showed the best clinical outcomes. These findings, presented at the Eastern Economic Forum and the St. Petersburg International Economic Forum, underscore Enteromix’s potential to shift the paradigm in cancer immunotherapy.
The AI Advantage: Accelerating Discovery and Personalization
Artificial intelligence is not merely a support tool for Enteromix; it is the engine driving unparalleled speed and precision:
- Neoantigen Selection Accuracy: Custom deep learning frameworks trained on vast immunopeptidomics datasets achieve over 90 percent accuracy in predicting which peptides will bind patient-specific HLA molecules and trigger potent T cell responses.
- mRNA Sequence Optimization: Generative models propose mRNA constructs with superior stability and translation profiles, reducing degradation and maximizing antigen expression in dendritic cells.
- Automated Manufacturing: Robotics guided by AI continuously adjust synthesis parameters, ensuring consistent mRNA capping efficiency and uniform LNP size distribution. Process analytics flag deviations in real time, preventing batch failures and accelerating scale-up.
This AI-driven integration compresses timelines from predicted weeks or months to mere days—an essential capability for treating aggressive cancers where time is of the essence.
Technical Blueprint: mRNA Constructs and LNP Formulations
Enteromix uses mRNA transcripts of 1,200 to 1,800 nucleotides encoding multiple neoantigens fused within a single open reading frame. The mRNA includes untranslated regions optimized for efficient ribosomal loading and modified nucleosides to evade innate immune sensors. Encapsulation occurs in LNPs comprising an ionizable lipid, distearoylphosphatidylcholine (DSPC), cholesterol, and a PEG-conjugated lipid at a 50:10:38.5:1.5 molar ratio. These LNPs are engineered to target antigen-presenting cells, facilitate endosomal escape, and protect the mRNA payload from extracellular RNases.
To further enhance stability, Enteromix explores RNA-plex technology, wherein mRNA strands are complexed with polymeric stabilizers, allowing extended storage at 2–8 °C and reducing dependence on ultra-cold chain logistics.
Manufacturing at Scale: From Bench to Bedside
Enteromix’s production facilities employ fully automated mRNA synthesizers coupled with AI-powered quality control modules. High-throughput reactors transcribe mRNA on demand, while inline spectroscopic sensors verify nucleotide incorporation and capping efficiency. Downstream microfluidic mixers combine mRNA with LNP components under controlled shear conditions, producing uniform nanoparticles. The entire process—from receiving sequencing data to dispatching a patient’s personalized vaccine—occurs within a tightly monitored seven-to-ten-day window, a dramatic improvement over conventional personalized therapy timelines.

Comparative Landscape: Enteromix vs. Global Competitors
While biotech leaders such as BioNTech and Moderna have developed their own mRNA cancer vaccine candidates, Enteromix differentiates itself through:
- Speed of Personalization: 7–10 days from biopsy to vaccine versus 3–6 weeks for competitor platforms.
- AI Integration: Advanced deep learning for neoantigen ranking and mRNA design, boosting immunogenic prediction accuracy.
- Enhanced Stability: Adoption of RNA-plex complexes to extend cold chain flexibility.
- Clinical Outcomes: Early Phase I results indicating 100 percent safety and over 48 percent combined complete/partial response rates, surpassing reported metrics from initial competitor trials.
Regulatory Pathway and Global Expansion
Following the successful Phase I trial, Enteromix is under review by Russia’s Ministry of Health for conditional approval. Plans are underway for Phase II/III studies in colorectal, glioblastoma, and melanoma patients, with trial sites to include leading oncology centers across Europe. Simultaneously, the development team is preparing submissions for WHO prequalification and engaging potential global manufacturing partners to ensure rapid, worldwide access upon approval.
Future Directions: Toward Universal Cancer Vaccines
Looking beyond Enteromix’s initial success, researchers envision next-generation mRNA vaccines employing circular RNA (circRNA) for prolonged antigen expression and self-amplifying RNA (saRNA) constructs to reduce dosing requirements. Additionally, studies are exploring shared neoantigen panels for semi-universal vaccines that can be deployed rapidly across patient cohorts. Combination regimens pairing Enteromix with checkpoint inhibitors or oncolytic viruses are also in development, aiming to overcome tumor immune evasion and achieve durable remissions.
Conclusion: The Dawn of AI-Powered Precision Immunotherapy
Enteromix represents a watershed moment in oncology: the seamless integration of personalized mRNA vaccine technology and artificial intelligence has yielded a platform capable of designing, manufacturing, and delivering bespoke cancer immunotherapies in days. Early clinical results—100 percent safety and significant tumor responses—validate this approach’s promise. As Phase II/III trials unfold and regulatory approvals expand globally, Enteromix may catalyze a broader shift from conventional therapies to precision immunotherapy, offering patients a tailored, effective, and safer alternative.

Disclaimer
This article is for informational purposes and does not constitute medical advice. Enteromix is under clinical investigation; its safety and efficacy require confirmation in larger, controlled trials. Consult qualified healthcare professionals for guidance on cancer treatment and clinical trial participation.



hi kindly share the source of results of this phase 1 data , iwas unable to find it anywhere on internet
The Phase I results for Enteromix were announced by Russia’s National Medical Research Radiology Centre (NMRRC) and presented at two high-profile forums in 2025—the St. Petersburg International Economic Forum (SPIEF) and the Eastern Economic Forum (EEF). As of now, detailed data have not yet been published in a peer-reviewed journal or posted on public clinical-trial registries. The primary sources are:
Official press releases from the Russian Ministry of Health and NMRRC presented at SPIEF 2025 and EEF 2025.
Kindly share the link of official press release detailing results of Phase data