Wearable AI Monitors Post-Op Recovery: Smart Patches for Early Infection Detection and Safer Discharge
Surgical care has increasingly shifted from long hospital stays to early discharge and outpatient recovery. While this benefits patients and health systems, it creates a monitoring gap: serious postoperative complications—especially surgical site infections (SSIs) and sepsis—often develop at home, where subtle warning signs may be missed until patients are seriously ill. SSIs remain one of the most common healthcare-associated infections worldwide, driving readmissions, reoperations, and costs.
Wearable AI-enabled wound patches are emerging to close this gap. By combining flexible biosensors, wireless electronics, and machine learning, “smart bandages” can continuously analyze wound exudate and local skin conditions, detect abnormal biomarker patterns, and notify clinicians or patients when infection risk is rising—often days before overt symptoms. Early evidence from preclinical and early human studies suggests these systems could transform post-op surveillance from sporadic visual checks into continuous, data-driven monitoring.
From Passive Dressings to Intelligent Post-Op Sensors
Traditional post-op dressings are passive: they protect incisions but provide no information between clinic visits. Clinicians rely on patients to notice redness, pain, or discharge and make contact, which often happens late.
Over the last decade, several technological trends have converged:
- Flexible biosensors that can conform to skin and measure pH, temperature, oxygen, moisture, uric acid, glucose, lactate, nitric oxide, and hydrogen peroxide directly from wound fluid.
- Battery-free and wireless platforms powered via NFC or inductive coupling, enabling lightweight, disposable patches suitable for home use.
- Edge AI and TinyML models that run inference locally or on paired mobile devices to interpret multivariate signals and classify healing status or infection risk in real time.
These technologies are now being integrated into postoperative smart patches designed specifically for surgical incisions and deep wounds.
What Do Post-Op Smart Patches Measure?
Research prototypes and early clinical devices typically monitor a panel of wound and systemic biomarkers known to change during infection or impaired healing:
- Temperature: Local wound temperature rises with inflammation and infection; several “soft intelligent dressings” include flexible temperature sensors for early warning.
- pH: Healthy wounds trend from alkaline toward neutral/acidic during healing; persistently alkaline pH often indicates infection or biofilm.
- Uric acid, NO, and H₂O₂: Elevated concentrations in exudate correlate with inflammation, bacterial activity, and oxidative stress in infected wounds.
- Moisture and exudate volume: Excess fluid, especially with changing composition, can indicate dehiscence or infection.
- Specific infection or sepsis biomarkers: For higher-risk patients, some systems monitor procalcitonin (PCT) in wound exudate as an early warning sign of sepsis.
For example:
- A battery-free wound dressing system was developed that wirelessly monitors local PCT levels through an NFC-powered patch to enable early sepsis diagnosis after contaminated or high-risk wounds.
- A conducting‑polymer “theranostic” bandage tracks pH and uric acid and releases ciprofloxacin on demand when abnormal levels suggest infection, controlled electrically and monitored remotely.
- Textile-based “smart bandaids” incorporate organic electrochemical transistors to continuously monitor uric acid in wound exudate across clinically relevant ranges, opening the way for real-time infection surveillance.
Post-op focused systems extend these same principles to surgical incisions in orthopedics, abdominal surgery, and plastic/reconstructive procedures, where early detection of superficial or deep SSI can alter the entire trajectory of recovery.
Where AI Comes In: Turning Raw Signals into Actionable Alerts
Raw sensor data alone do not guarantee safer recovery; clinicians need interpretable, clinically meaningful information. AI models fill this gap in several ways.
1. Classifying Healing Stage and Abnormal Recovery
The PETAL patch (a paper-like battery-free multiplex sensor) combines colorimetric chemistries for pH, temperature, oxygen, and inflammatory markers with a deep neural network that maps multimodal sensor patterns to wound healing stages.
- In preclinical work, PETAL’s ANN-based classifier recognized healing phases (e.g., inflammatory vs proliferative) and abnormal trajectories with high accuracy, enabling automated identification of non-healing or deteriorating wounds.
- A separate FLEX-AI contactless wearable sensor system used a deep ANN trained on pH-responsive voltage outputs to classify healing stages in chronic wounds, achieving 94.6% accuracy.
For post-op patients, similar architectures can be trained on surgical incision data to distinguish:
- Expected early inflammation vs. pathological inflammation.
- Normal healing vs. patterns that historically precede SSI or dehiscence.
- Stable vs. deteriorating trajectories over days.
2. Detecting Infection Days Before Symptoms
Caltech’s iCares smart bandage provides a concrete example of biomarker‑plus‑AI monitoring in human wounds:
- Microfluidic channels continuously sample fresh wound exudate while clearing excess moisture.
- Sensors measure nitric oxide (inflammation marker) and hydrogen peroxide (infection marker), along with pH and temperature.
- A machine‑learning model trained on longitudinal patient data classifies wound status and predicts healing time with AUC 0.9–0.92, comparable to expert clinicians, and detects inflammatory/infectious signatures one to three days before symptoms appear.
Although these initial trials target chronic wounds, the same biomarkers and analytic logic are directly relevant to postoperative incisions, which follow similar inflammatory and repair phases and are vulnerable to bacterial contamination.
3. Closed-Loop Therapy: Not Just Monitoring, But Intervention
A stretchable wireless bioelectronic bandage reported in Science Advances takes the concept further: it not only monitors multiple wound biomarkers (pH, temperature, glucose, lactate, uric acid) but also performs on-demand antimicrobial drug release and electrical stimulation to accelerate healing in infected diabetic ulcers.
- Multiplexed monitoring revealed spatial and temporal changes in wound microenvironments.
- AI-like control logic coordinated drug release and electrical cues to produce substantially accelerated chronic wound closure in rodents compared with standard dressings.
Similarly, a new AI-powered device called a‑Heal uses a tiny camera and machine-learning algorithms to detect the current healing stage of a wound and deliver tailored medicine or electrical fields, achieving about 25% faster healing than standard care in preclinical tests.
Conceptually, post-op patches could operate in the same closed-loop fashion:
- Sense → interpret via AI → alert clinicians and/or automatically adjust local therapy (e.g., antibiotic release, negative pressure parameters, or stimulation) for high-risk incisions.
Evidence That AI Can Spot Post-Op Infections Early
Alongside bandage-based systems, several groups are using AI on other postoperative data streams—showing that infection signatures are detectable well before standard diagnosis.
EHR-Based Early Infection Prediction
The PERISCOPE multicentre study (Lancet Regional Health Europe) developed AI models using routine EHR data to detect postoperative infections earlier than traditional criteria.
- Models trained on vital signs, lab values, and clinical documentation outperformed standard scoring rules for early post-op infection recognition.
- This demonstrates that pattern-recognition AI can extract early infection signals from noisy, real-world data, an encouraging precedent as similar models are adapted to high-frequency biosensor data from patches.
Image-Based SSI Detection from Patient Photos
Mayo Clinic researchers created an AI pipeline that detects SSIs directly from patient-submitted smartphone photos of postoperative wounds:
- Stage 1: a deep-learning model identifies whether an image contains a surgical incision.
- Stage 2: another model evaluates that incision for signs of infection.
- Trained on >20,000 images from >6,000 patients, the system achieved 94% accuracy for incision detection and an AUC of 0.81 for infection identification.
While this is image-based rather than patch-based monitoring, it shows that AI can robustly classify infection status remotely, making it natural to combine:
- Visual data from patients’ phones, and
- Continuous biochemical data from smart patches,
into a more comprehensive post-op surveillance system.
Potential Impact on Postoperative Care Pathways
1. Safer Early Discharge and Virtual Follow-Up
Smart patches can transmit data to a patient’s phone and onward to the hospital:
- At discharge, the patch is applied over the incision with a clear wear-and-replace schedule.
- At home, the patch streams biomarker and contextual data (e.g., local temperature, exudate markers).
- AI triage models classify risk (e.g., green/yellow/red) and push alerts to both patient and care team dashboards when patterns deviate from expected trajectories.
This supports:
- Early detection of SSIs and sepsis risk before patients feel “sick.”
- Prioritization of high-risk patients for urgent review, while low-risk patients can safely continue standard virtual follow-up.
- Reduced burden on surgical teams who would otherwise review large volumes of routine pictures or messages manually.
2. Reduced Readmissions and Complications (Projected)
Direct randomized evidence in postoperative populations is still emerging, and most outcome data so far are from chronic wound models. However:
- Smart bandage systems have accelerated wound closure and improved infection control in preclinical and early clinical settings.
- Early detection of infection and sepsis biomarkers (e.g., PCT) via wearable dressings is expected to reduce delayed diagnoses, which are a key driver of morbidity and readmission after surgery.
- Reviews of smart dressings for surgical wounds highlight the strong theoretical and preclinical basis for reducing SSI-related complications; the main gap is large-scale clinical validation in post-op patients, which several groups and at least one registered study on wearables for early postoperative detection are beginning to address.
Given that SSIs and related complications substantially increase length of stay and costs, even modest improvements in early detection could have outsized clinical and economic impact.
Current Limitations and Challenges
Despite the promise, there are important caveats:
- Most human data are in chronic, not acute post-op wounds. Translation to surgical incisions is ongoing, and performance will need to be validated across different surgeries and patient populations.
- Sensor stability and calibration over several days of wear remain technical challenges, especially for multi-analyte systems that must operate under motion, sweat, and varying temperatures.
- False positives and alert fatigue are real risks; thresholds and AI models must be tuned to balance early warning with specificity, and to integrate with clinician workflows rather than overwhelm them.
- Regulatory and integration hurdles include:
- Demonstrating safety and accuracy in prospective trials.
- Integrating continuous data streams into EHRs and clinical decision support.
- Defining reimbursement models for remote post-op monitoring.
Ethically, systems must be designed to work across different skin tones, body habitus, and care settings, and to avoid widening digital divides (e.g., for patients without smartphones or stable connectivity). Reviews of intelligent patches stress the need for robust regulation and attention to sensor precision, resilience, and data governance before wide clinical deployment.
Future Directions: From “Smart Dressings” to Integrated Post-Op Platforms
Over the next several years, multiple trends are likely to converge:
- Richer multimodal data: Combining biochemical patch data, smartphone images, patient-reported symptoms, and backend EHR signals into unified AI risk scores.
- Personalized baselines: Using each patient’s early post-op data to establish their own “normal” pattern, so deviations are detected more sensitively than with one-size-fits-all thresholds.
- Closed-loop therapy: Expanding systems like the stretchable theranostic bandage and a‑Heal to post-op wounds, where patches not only detect risk but adjust local therapy (e.g., heat, antibiotics, electrical stimulation) under clinician-defined protocols.
- Deep and semi‑implantable devices for complex surgeries: Devices that monitor deep tissue biomarkers via microchannels or semi-implantable components are already being explored for deep wounds and surgical sites, with multiplex biochemical testing and AI‑supported interpretation.
As these capabilities mature, the standard of care for post-op follow-up may shift from infrequent, subjective visual checks to continuous, quantitative, AI-interpreted monitoring.
AI Wearable Post-Op Monitoring Loop
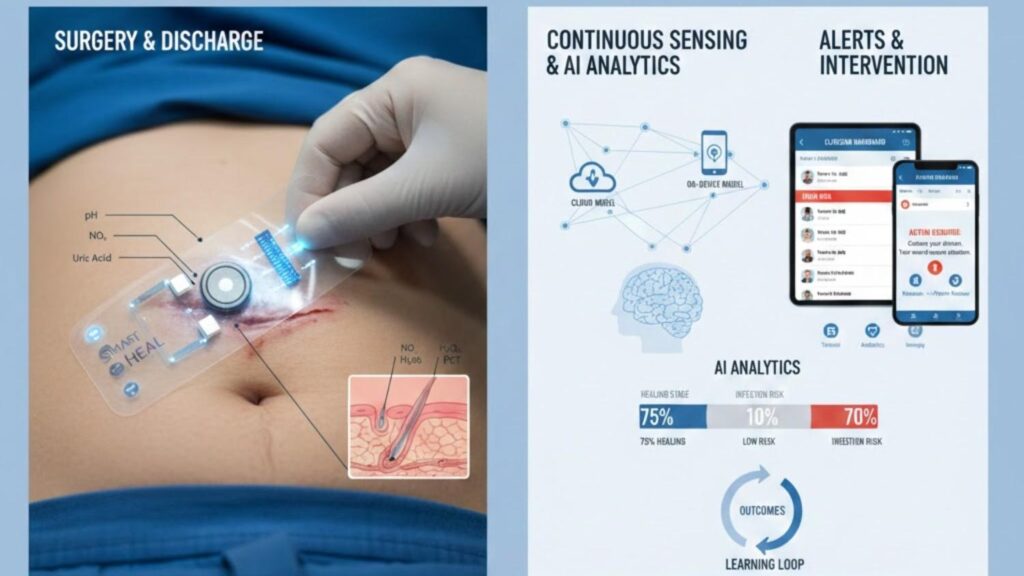
Conclusion: Toward Safer, Smarter Recovery at Home
Wearable AI patches for wound monitoring are moving from concept to clinic. By continuously analyzing biomarkers directly at the incision site and applying machine learning to detect abnormal trajectories, these systems promise to:
- Catch infections and sepsis risk earlier, often days before symptoms.
- Support safe early discharge and virtual follow-up.
- Reduce avoidable readmissions and complications.
- Give surgeons objective, continuous insight into how their patients are healing at home.
The strongest data so far come from chronic wound and preclinical models, but the same architectures—smart biosensors plus AI analytics—are now being adapted and trialed for postoperative recovery. As robust clinical evidence accumulates and integration challenges are addressed, AI-enabled smart patches are poised to become a core component of post-op care pathways, turning every dressing into a data-rich, proactive monitoring tool rather than a passive cover.




This seems like a breakthrough in bridging the monitoring gap after surgery. Rather than waiting for visible signs of infection, having continuous data-driven insights would provide so much more confidence to both patients and clinicians.