ICH GCP E6(R3) Finalized on January 6, 2025, Includes DCT, e-Consent, and Artificial Intelligence
The E6(R3) update, finalized on January 6 2025 (Step 4 of the ICH process), is the most significant overhaul since the 2016 E6(R2) revision. It reflects advancements in clinical trial methods, technology, and regulatory expectations over nearly a decade.
Why it matters:
- Trials are increasingly decentralized and technology-driven.
- Regulators demanded clearer standards on data governance, participant engagement, and oversight.
- Sponsors and investigators needed flexible, risk-based approaches without compromising safety or quality.
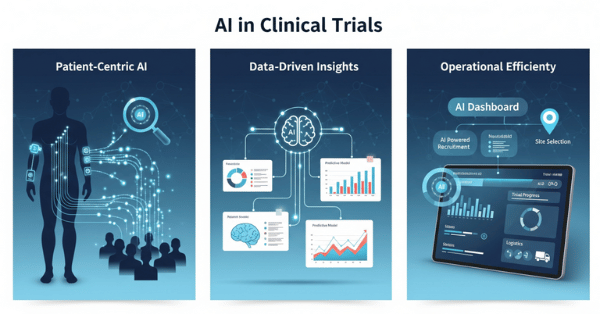
Real-World Applications
- Multinational Trials: Large pharmaceutical companies can now use centralized monitoring and remote data verification as compliant strategies.
- Patient-Centric Studies: Home delivery of investigational products and eConsent platforms now have explicit support in the guidelines.
- AI-Driven Data Analysis: The new Data Governance section acknowledges digital and AI-based trial data handling.
Latest Research or Updates
The E6(R3) final document consists of:
- Core Principles: Broad, foundational statements applicable to all clinical trials.
- Annex 1: Detailed requirements for interventional trials with human participants.
- Annex 2: Still under public consultation (as of early 2025) for non-traditional trial types like pragmatic or low-intervention studies.
Key innovations include:
- Expanded definitions (e.g., “service provider” replacing “CRO” to capture all third parties).
- ALCOA+ principles for data integrity.
- Risk-proportionate approaches embedded from trial design through monitoring.
Applications or Implications
- Regulators: Can enforce a modernized, harmonized set of GCP principles across jurisdictions, reducing duplication of compliance efforts.
- Sponsors: Greater flexibility to adopt innovative technologies without waiting for regulatory variances.
- Sites: Better clarity on responsibilities and recordkeeping requirements, especially in hybrid or remote trials.
- Participants: Improved protection through better consent processes and data safeguards.
Challenges or Limitations
- Transition Period: Organizations must train staff, update SOPs, and modify trial documentation to comply.
- Global Alignment: While ICH members adopt E6(R3), local regulations may lag, causing interim compliance complexity.
- Annex 2 Uncertainty: Some trial types won’t have finalized detailed guidance until Annex 2 is complete.