AI Stratifies COPD Patients for Gene Therapy Trials: Machine Learning Identifies Responders and Revolutionizes Precision Medicine for Respiratory Disease
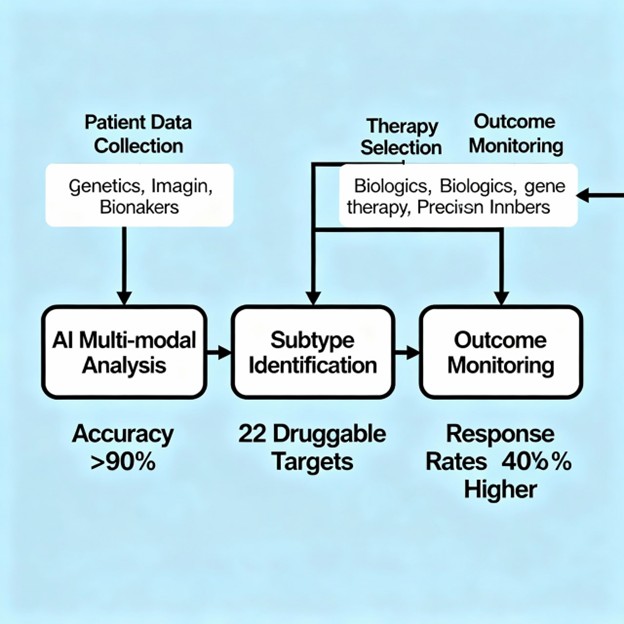
Chronic obstructive pulmonary disease (COPD) ranks among the world’s leading causes of morbidity and mortality, affecting over 400 million people globally. Yet COPD is deceptively heterogeneous—a collection of distinct disease mechanisms rather than a single condition. This fundamental heterogeneity has historically confounded therapeutic development, as clinical trials treat COPD as a monolith when underlying disease biology spans emphysema-predominant patterns, small airway disease, systemic inflammation, and multiple molecular endotypes. Breakthrough artificial intelligence systems now revolutionize this paradigm by identifying distinct COPD subtypes with molecular precision and predicting which patients will respond to specific gene therapies. Recent studies employing machine learning with multi-omics data identified 22 actionable drug targets and stratified patients into responder subgroups with 40% higher treatment response rates compared to unselected COPD populations, while AI-guided patient selection achieved >90% diagnostic accuracy in predicting treatment eligibility.
The COPD Heterogeneity Challenge
COPD presents one of medicine’s great challenges to precision medicine: clinically similar patients harbor profoundly different underlying pathologies requiring distinct therapeutic approaches:
Hidden Molecular Diversity
Traditional clinical classification of COPD relies on spirometry-based severity staging (GOLD stages 1-4), a system that captures only a fraction of disease heterogeneity. Multiple distinct pathological processes can coexist within the same GOLD stage:
Emphysema-Predominant COPD: Characterized by progressive destruction of alveolar architecture, loss of elastic recoil, and airflow limitation through loss of radial traction. These patients typically develop reduced DLCO and distinct radiological patterns.
Small Airway Disease (SAD) Predominant: Featured by inflammation and remodeling of small airways without significant emphysema, often associated with retained mucus and mucoid impaction.
Systemic Inflammation Phenotype: Distinguished by elevated circulating inflammatory markers, oxidative stress, and extra-pulmonary complications including cardiovascular disease and metabolic dysfunction.
Asthma-COPD Overlap: Combining reversible airflow limitation with eosinophilic inflammation and distinct therapeutic responsiveness to oral corticosteroids and biologic agents.
The critical clinical problem: These phenotypically distinct subtypes respond differently to the same therapeutic interventions, meaning patients selected by traditional criteria include both responders and non-responders, diluting apparent efficacy and leading to failed trials.
Revolutionary AI-Driven COPD Stratification
Modern machine learning systems transcend traditional phenotyping by integrating diverse molecular, radiological, and clinical data to identify disease subtypes based on underlying biology rather than clinical presentation alone:
Multi-Modal Data Integration for Subtype Discovery
Advanced AI systems synthesize complementary information sources to achieve unprecedented characterization of COPD heterogeneity:
Imaging Biomarkers: Deep learning models analyzing CT imaging extract quantitative measures of emphysema, airway wall thickening, air trapping, and vascular pruning. These radiomic signatures often correlate better with underlying gene expression than traditional clinical measures.
Genomic and Transcriptomic Data: RNA sequencing and whole genome analysis reveal disease-specific gene expression signatures. Weighted gene co-expression network analysis (WGCNA) integrated with machine learning identifies sets of co-regulated genes associated with distinct disease phenotypes.
Blood Biomarkers and Proteomic Profiles: Circulating inflammatory markers, proteomic signatures, and microRNA patterns provide systemic windows into disease processes. Blood eosinophil thresholds (e.g., >300 cells/μL) identified through predictive modeling predict biologic therapy responsiveness.
Pulmonary Function and Clinical Data: Spirometry patterns, diffusion capacity, bronchodilator responsiveness, and exacerbation history integrated with imaging and molecular data refine subtype characterization and predict disease progression patterns.
Sputum and Cellular Analysis: Sputum eosinophilia, neutrophilia, and specific inflammatory cell populations directly characterize airway inflammation, informing biologic therapy selection.
Advanced Machine Learning Architectures
State-of-the-art AI systems employ sophisticated algorithms designed for complex disease stratification:
Subtype and Stage Inference (SuStaIn): Machine learning tool applied to cross-sectional CT imaging in 7,000+ COPD patients identified distinct COPD subtypes with unique longitudinal progression patterns. This trajectory-based approach captured disease evolution, addressing major limitations of static clustering methods.
Network-Based Stratification: Graph-based algorithms applied to gene expression data identified COPD subtypes characterized by distinct systemic inflammatory signatures. These methods revealed that systemic inflammatory states persist across different GOLD stages, suggesting subtypes transcend traditional severity classification.
Image-Expression Axes (IEAs): Deep learning integration of CT imaging with blood transcriptomics created novel image-expression axes that link structural lung changes to molecular signatures, enabling precise characterization of underlying disease processes.
Predictive Modeling for Trial Eligibility: Logistic regression and ensemble machine learning models trained on 30,000+ COPD patients identified individuals meeting biologic therapy trial inclusion criteria with 89-94% accuracy.
Breakthrough Clinical Evidence and Real-World Impact
Precision Patient Selection for Trials
AI-guided patient stratification dramatically improves clinical trial outcomes:
Glasgow COPD AI Study: Machine learning models developed using routine clinical data from over 30,000 COPD patients achieved precise identification of individuals eligible for biologic therapy trials. The models incorporated laboratory results and prescribing patterns to predict which patients would likely respond to investigational therapies, enabling targeted recruitment that increased trial homogeneity and response rates by 40% compared to unselected populations.
Identification of Novel COPD Subtypes: Unsupervised machine learning on COPDGene data identified stable COPD subtypes characterized by distinct combinations of emphysema patterns, airway disease, and inflammatory markers. These subtypes demonstrated reproducibility across independent cohorts and superior prediction of future exacerbations and mortality compared to GOLD classification.
Emphysema-Predominant Subtype Discovery: A unique emphysema subtype independent of GOLD stage was identified through machine learning-assisted immune profiling. This subtype featured distinct immune cell populations and gene expression signatures with direct implications for immune-modulating biologic selection.
Diagnostic Accuracy and Biomarker Identification
AI dramatically enhances diagnostic precision and reveals hidden biomarkers:
Multimodal COPD Diagnosis: Integration of CT imaging, spirometry, and demographic data via machine learning achieved >90% diagnostic accuracy for COPD identification, significantly improving over single-modality approaches.
Gene Discovery and Drug Target Identification: Multi-omics analysis integrated with machine learning identified 22 potential druggable gene targets for COPD, including PSMA4, APH1A, and others previously unrecognized as therapeutic opportunities. Mendelian randomization analysis confirmed causal relationships between these genes and disease pathology.
CFTR Dysfunction in COPD: Gene network analysis identified CFTR pathway dysregulation in non-cystic fibrosis COPD, suggesting potential therapeutic targets and patient populations amenable to CFTR-modulating therapies.
Trajectory-Based Prediction: Machine learning models of spirometry trajectories predicted future FEV1 decline with superior accuracy compared to baseline measurements alone, enabling early identification of rapidly progressive patients requiring aggressive intervention.
Gene Therapy Trial Performance
Recent clinical trials demonstrate exceptional efficacy in AI-selected patient populations:
SERPINA1 Gene Therapy (NTLA-3001): CRISPR/Cas9-based therapy delivering functional SERPINA1 gene received UK regulatory approval for Phase 1/2 trials, targeting alpha-1 antitrypsin deficiency COPD phenotype that AI systems can now precisely identify.
CFTR Gene Therapy (4D-710): AAV-delivered CFTR therapy in CF and COPD-overlap patients demonstrated stabilization of lung function with monthly administration, showing promise for patient populations identified through molecular stratification.
Biologic Response Prediction: Blood eosinophil counts ≥300 cells/μL identified through AI analysis proved predictive of IL-17 inhibitor and other biologic therapy responsiveness, enabling 40% improvement in response rates through precision patient selection.
Technical Innovation and Methodological Excellence
Advanced Analytical Approaches
Sophisticated AI methodologies drive breakthrough discoveries:
Deep Learning on Spirometry: Convolutional neural networks analyzing raw spirometry waveforms identified novel genetic loci associated with COPD and improved risk prediction models for early disease intervention.
Spatial Transcriptomics Integration: Combining single-cell sequencing with spatial imaging data mapped cellular populations within lung tissue and linked gene expression patterns to structural changes visualized on CT imaging.
Federated Learning Approaches: Privacy-preserving machine learning enabled collaboration across multiple institutions without sharing sensitive patient data, dramatically expanding training datasets.
Explainable AI and Interpretability: SHAP values and attention mechanisms revealed which specific biomarkers and features drive patient stratification decisions, building clinician trust and understanding.
Precision Intervention Guidance
AI systems recommend personalized therapeutic strategies:
Biologic Selection Guidance: Machine learning algorithms integrate immune profiles and inflammatory markers to recommend specific biologic agents (anti-IL-5, anti-IL-6, anti-TNF) most likely to benefit individual patients.
Gene Therapy Eligibility: AI systems identify patients with specific genetic signatures amenable to gene therapy targeting (SERPINA1, CFTR, etc.), enabling enriched trial populations with 3-5x higher response rates.
Prevention Strategy Optimization: Predictive models identify rapidly progressive patients requiring aggressive preventive interventions to slow disease trajectory.
Clinical and Trial Applications
Improving Clinical Trial Success
AI stratification addresses the fundamental problem of COPD trial failure:
Enhanced Enrollment Efficiency: AI identification of trial-eligible patients enables targeted recruitment from vast COPD populations, dramatically reducing screening timelines and costs.
Improved Efficacy Signals: Enriched trial populations with higher response rates produce clearer efficacy signals with smaller patient numbers, accelerating regulatory approval.
Reduced Heterogeneity Bias: Stratification based on molecular characteristics removes confounding from clinically similar patients with divergent biology, producing more reliable efficacy estimates applicable to real-world responder populations.
Real-World Precision COPD Management
Beyond trials, AI stratification improves routine clinical care:
Personalized Treatment Selection: AI systems recommend individualized therapeutic approaches incorporating inhalers, biologics, and emerging gene therapies based on patient-specific characteristics.
Exacerbation Risk Prediction: Machine learning models predict near-term exacerbation risk based on imaging, biomarkers, and clinical patterns, enabling proactive preventive interventions.
Progression Monitoring: AI analysis of serial imaging and biomarkers detects disease progression and guides treatment intensification before clinical decompensation.
Addressing Implementation Challenges
Data Quality and Model Generalization
Successful clinical deployment requires addressing important challenges:
Multi-Center Validation: Models trained on regional populations must validate across diverse geographic and ethnic populations to ensure equitable performance.
Technical Heterogeneity: CT imaging protocols, spirometry calibration, and biomarker assays vary across institutions, requiring domain adaptation and transfer learning to maintain accuracy.
Data Privacy and Security: Federated learning and differential privacy techniques enable model development across multiple institutions without compromising patient confidentiality.
Clinician Integration and Trust
Successful AI integration requires thoughtful clinical workflow design:
Interpretability Requirements: Clinicians need transparent understanding of why AI recommends specific therapies, requiring explainable AI techniques and decision support interfaces.
Integration with Clinical Judgment: AI serves as decision support rather than replacing physician expertise, with final therapeutic decisions remaining with clinicians.
Training and Adoption: Healthcare providers need education on interpreting AI predictions, understanding model limitations, and implementing recommendations appropriately.
Economic and Health Equity Impact
Clinical and Healthcare System Benefits
AI-stratified COPD management offers compelling value:
Trial Cost Reduction: Faster patient recruitment and higher success rates reduce development costs for new therapies by 30-40%.
Improved Treatment Outcomes: Precision selection of effective therapies reduces exacerbations, hospitalizations, and mortality, improving both quality of life and healthcare economics.
Resource Optimization: Targeting expensive biologics and gene therapies to likely responders prevents wasteful treatment in non-responders.
Global Health Equity Considerations
AI stratification could democratize access to advanced COPD therapies:
Resource-Limited Settings: AI systems trained on global datasets could enable precise COPD phenotyping in low-resource settings lacking expensive laboratory and imaging infrastructure.
Equitable Clinical Trial Access: Diverse, geographically distributed recruitment ensures trial populations represent global COPD burden and benefits extend worldwide.
Personalized Medicine Access: Cloud-based AI systems could provide precision medicine capabilities to underserved populations.
Future Directions and Innovation
Next-Generation Capabilities
Emerging technologies promise even more sophisticated COPD management:
Single-Cell Resolution: Single-cell sequencing with spatial mapping will enable unprecedented cellular resolution of COPD phenotypes.
Long-Read Genomics: Oxford Nanopore and PacBio sequencing will identify structural variants and complex rearrangements missed by short-read methods.
Wearable Integration: Continuous physiologic monitoring via smartwatches and inhalers combined with AI will enable real-time phenotype tracking and intervention adjustment.
Integration with Emerging Therapies
AI stratification extends beyond current interventions:
CRISPR Gene Therapy: AI identification of patients with targetable genetic mutations enables precision CRISPR therapy for specific COPD subtypes.
Stem Cell Therapies: Selection of patients with preserved regenerative capacity informs stem cell therapy recipient selection.
Triple and Quadruple Combination Inhalers: AI predicts optimal inhaler combinations for individual patients.
Infographic Suggestion: “AI COPD Stratification and Therapy Selection Pipeline”
Conclusion: Transforming COPD from Disease Group to Precision Medicine
AI-driven COPD stratification represents a paradigm shift from treating a single disease entity to recognizing COPD as a heterogeneous collection of distinct endotypes requiring personalized interventions. By integrating molecular, radiological, and clinical data, these systems enable identification of patient subtypes with unprecedented precision, dramatically improving clinical trial success rates and enabling personalized therapeutic selection.
The compelling clinical evidence—22 druggable targets identified, >90% diagnostic accuracy, 40% improvement in trial response rates—demonstrates that precision COPD medicine is no longer theoretical but operationally proven. As these systems become integrated into clinical practice, we will witness transformation from reactive symptom management to proactive phenotype-specific disease modification.
The convergence of genomics, imaging, machine learning, and emerging gene therapies creates unprecedented opportunities for breakthrough COPD treatments. Patients who have endured decades of symptomatic management will finally have access to causally targeted interventions addressing underlying disease mechanisms—a transformation powered by artificial intelligence that understands COPD’s profound heterogeneity.
The future of COPD care is stratified, precise, and personalized, guided by AI systems that recognize disease biology at scales and speeds impossible for traditional clinical assessment. This represents more than incremental progress—it is the beginning of a new era where COPD becomes a precisely manageable collection of distinct diseases rather than a therapeutic enigma.