The Global Burden of Disease Study: Origins, Evolution, and the Future with Artificial Intelligence
The Global Burden of Disease (GBD) Study represents one of the most significant advances in public health measurement of the past three decades. This comprehensive epidemiological initiative has fundamentally transformed how the global health community quantifies, prioritizes, and addresses health challenges worldwide.
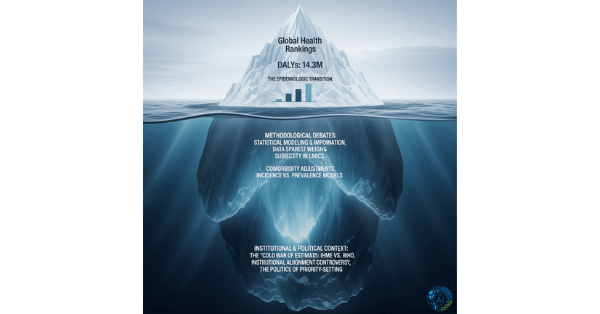
What is the Global Burden of Disease Study?
The GBD is the world’s most extensive scientific undertaking for systematically measuring and comparing population health across time and geographic regions. Think of the GBD as the world’s most detailed health report card. At its core, the study employs a standardized framework to quantify health loss using the Disability-Adjusted Life Year (DALY), a metric that combines two critical components:
- Years of Life Lost (YLL): Quantifies premature mortality by measuring life years lost due to death before reaching standard life expectancy
- · Years Lived with Disability (YLD): Captures the burden of living with illness or disability by weighting time spent in suboptimal health states
The DALY formula is elegantly simple yet comprehensive: DALY = YLL + YLD. One DALY represents the equivalent of losing one year of healthy life, enabling direct comparisons between vastly different health conditions and their impacts across populations.
Historical Context and the Need for Standardized Health Measurement
Before the 1990s, global health decision-making relied heavily on fragmented mortality statistics and politically influenced advocacy rather than systematic evidence. This approach had critical limitations:
- Mortality bias: Death counts dominated health discourse while non-fatal conditions remained largely invisible in policy discussions
- Inconsistent metrics: Different organizations used varying methodologies, making meaningful comparisons impossible
- Advocacy distortion: Disease burden claims often reflected political priorities rather than empirical evidence
The absence of standardized measurement meant that conditions like depression, arthritis, and back pain—which cause substantial disability but limited mortality—received inadequate attention in global health agendas.
Evolution and Major Milestones
The Founding Era (1990-1993)
The original GBD study was commissioned in 1992 by the World Bank for its landmark “World Development Report 1993: Investing in Health”. Led by Christopher Murray at Harvard University and Alan Lopez at the World Health Organization, this inaugural effort analyzed 107 diseases and injuries across eight world regions. The study introduced revolutionary findings, including predictions that non-communicable diseases would become dominant health challenges even in developing countries—a forecast that proved remarkably prescient.
WHO Institutionalization (1998-2007)
Following the initial study’s impact, the World Health Organization established a dedicated division for global burden of disease estimation in 1998. WHO produced updated estimates for 2000, 2001, and 2002, but methodological limitations and resource constraints hindered comprehensive updates.
IHME Leadership Era (2007-Present)
A transformative shift occurred in 2007 with the establishment of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington. Funded by a $105 million grant from the Bill & Melinda Gates Foundation, IHME became the coordinating center for GBD research under Murray’s direction. This transition enabled:
- Massive scale expansion: From 107 conditions to 371 diseases and injuries in the latest iteration
- Enhanced geographic coverage: Comprehensive analysis of 204 countries and territories
- Risk factor integration: Assessment of 88 distinct risk factors contributing to disease burden
- Collaborative network growth: Over 7,700 scientists from 156 countries participate in the current GBD research
Current Scope and Methodology
The GBD 2021 represents the study’s most comprehensive iteration, employing sophisticated statistical modeling to address data gaps in regions with limited health surveillance. The study utilizes:
- Advanced Bayesian techniques: DisMod-MR 2.1 modeling ensures consistency across epidemiological parameters
- Diverse data integration: Vital registration systems, household surveys, disease registries, and satellite imaging contribute to estimates
- Uncertainty quantification: 95% uncertainty intervals acknowledge methodological and data limitations
- Annual updates: Regular estimation cycles provide current evidence for policy-making
Real-World Applications and Impact
GBD findings have profound implications across multiple domains:
Policy Formulation
Governments worldwide use GBD estimates to allocate health budgets based on quantified disease burden rather than political pressure. For example, GBD data demonstrating that low back pain and depression rank among leading causes of disability globally has influenced national health programs to prioritize mental health and musculoskeletal conditions.
Research Prioritization
The framework guides clinical trial design and funding decisions by identifying high-burden conditions with inadequate therapeutic options. Pharmaceutical companies increasingly reference GBD estimates when evaluating market opportunities and research investments.
Global Health Advocacy
By providing standardized metrics, GBD enables evidence-based advocacy for neglected conditions. The study’s revelation that neuropsychiatric disorders cause substantial global burden helped elevate mental health on international policy agendas.
Interactive Tools and Data Accessibility
IHME has developed comprehensive digital platforms to democratize access to GBD findings:
- GBD Compare: Interactive visualization tool enabling customized data exploration through treemaps, world maps, and trend charts
- GBD Results Tool: Comprehensive database allowing searches across causes, locations, and time periods
- Global Health Data Exchange (GHDx): Repository providing access to underlying data sources and methodological documentation
These platforms transform complex epidemiological data into accessible formats for researchers, policymakers, and public health practitioners globally.
The Role of Artificial Intelligence in GBD’s Future
Artificial intelligence presents transformative opportunities to enhance GBD’s accuracy, timeliness, and scope through several key applications:
Advanced Data Integration and Processing
Machine learning algorithms can synthesize diverse health data streams in real-time, including electronic health records, genomic databases, and wearable device outputs. This capability enables:
- Automated data quality assessment: AI systems can identify inconsistencies and biases in health data sources
- Multi-modal data fusion: Integration of imaging, genomic, and clinical data for more precise burden estimates
- Real-time surveillance: Continuous monitoring of disease trends through digital health platforms
Predictive Modeling and Forecasting
AI-powered predictive analytics can enhance GBD’s forecasting capabilities by:
- Disease trajectory modeling: Machine learning can predict epidemic patterns and chronic disease progression
- Policy scenario analysis: AI models can simulate health outcomes under different intervention strategies
- Resource optimization: Predictive algorithms can guide health system planning and resource allocation
Natural Language Processing Applications
NLP technologies can mine scientific literature, clinical notes, and social media for early disease signals:
- Literature synthesis: Automated extraction of epidemiological data from research publications
- Outbreak detection: Real-time monitoring of disease mentions across digital platforms
- Clinical data extraction: Automated processing of unstructured medical records
Computer Vision for Health Assessment
AI-powered image analysis can improve disease prevalence estimates in data-scarce regions:
- Medical imaging analysis: Automated diagnosis from radiological and pathological images
- Satellite-based health assessment: Analysis of environmental factors affecting disease transmission
- Mobile health applications: Smartphone-based diagnostic tools for remote health assessment
Examples of AI Implementation in Healthcare
Several successful AI applications demonstrate the technology’s potential for GBD enhancement:
- Diagnostic accuracy: Stanford University’s deep learning models achieve dermatologist-level performance in skin cancer detection
- Drug discovery acceleration: Pharmaceutical companies like Pfizer use AI to reduce development timelines
- Genomic analysis: Deep Genomics employs machine learning to identify disease-linked genetic mutations
- Predictive healthcare: Companies like Tempus use AI to extract clinical insights from unstructured medical data
Challenges and Limitations
Despite AI’s promise, several challenges must be addressed for successful GBD integration:
Technical Barriers
- Data standardization: Inconsistent formats across global health systems complicate AI implementation
- Computational requirements: Advanced AI models demand significant computing resources
- Algorithm bias: Machine learning systems may perpetuate existing health disparities
Ethical Considerations
- Privacy protection: Patient data security becomes more complex with AI-powered analysis
- Algorithmic transparency: “Black box” AI systems may lack interpretability for policy decisions
- Equity concerns: AI benefits may disproportionately favor well-resourced health systems
Future Directions and Opportunities
The integration of AI with GBD methodology promises several transformative developments:
Enhanced Precision
AI can improve DALY estimation accuracy in low-resource settings by leveraging proxy indicators and satellite data to infer health outcomes where direct measurement is impossible.
Real-Time Monitoring
Machine learning systems can provide continuous disease burden updates rather than periodic estimates, enabling rapid response to emerging health threats.
Personalized Population Health
AI can enable more granular analysis of disease burden across demographic subgroups, supporting targeted interventions for vulnerable populations.
Predictive Prevention
By identifying populations at risk before disease onset, AI-enhanced GBD could shift focus from treatment to prevention, potentially reducing global health burden.
Conclusion
The Global Burden of Disease Study has evolved from an innovative research project into an indispensable foundation of global health evidence. From its origins addressing 107 conditions in eight regions to its current comprehensive analysis of 371 diseases and injuries across 204 countries, the GBD has consistently expanded its scope and refined its methodology.
The study’s core innovation—the DALY metric—remains as relevant today as when first introduced, providing a common currency for comparing diverse health challenges. By quantifying both mortality and morbidity in a single measure, the GBD has enabled evidence-based priority-setting that transcends political boundaries and advocacy pressures.
As artificial intelligence technologies mature, their integration with GBD methodology promises to enhance the study’s accuracy, timeliness, and policy relevance. Through advanced data integration, predictive modeling, and automated analysis, AI can help ensure that no disease, condition, or community remains invisible in global health planning.
The future GBD, powered by artificial intelligence, will likely provide real-time health intelligence that enables proactive rather than reactive health policy. This evolution from periodic reporting to continuous monitoring represents the next frontier in global health measurement—one that promises to make health decisions faster, more precise, and more equitable for populations worldwide.


The sentences have a natural pulse, like breath or heartbeat. They create rhythm and presence, turning the act of reading into a meditative experience.